The Sinopharm COVID-19 vaccine is the sixth vaccine authorized by the World Health Organization for emergency use with its quality, safety and efficacy validated. To help more stay safe and protected against COVID-19, Farrer Park Hospital has received approval to administer the vaccine under the Special Access Route. If you would like to receive the vaccine, register your interest and we will get in touch.
The Sinopharm COVID-19 vaccine – scientifically known as SARS-CoV-2 Vaccine (VeroCell) – is an inactivated vaccine against COVID-19. Unlike Pfizer-BioNTech/Cominarty or Moderna mRNA vaccines where weakened or inactivated germs are used, the Sinopharm vaccine is made of inactivated virus. Once introduced into the body, the immune system starts to produce antibodies that fight against the SARS-CoV-2 coronavirus. The Sinopharm vaccine is adjuvanted with aluminium hydroxide to boost the response of the immune system.

According to results from clinical trials, common side effects after administration of the vaccine include:
According to the WHO, the vaccine is suitable for the following individuals:
 Aged 18 and above
Aged 18 and above
 No known history of anaphylaxis
No known history of anaphylaxis
Reference:
World Health Organization. 2021. COVID-19 Vaccine Explainer: COVID-19 Vaccine (Vero Cell), Inactivated (Sinopharm). Retrieved from: https://www.who.int/publications/m/item/sinopharm-vero-cell---inactivated-covid-19-vaccine
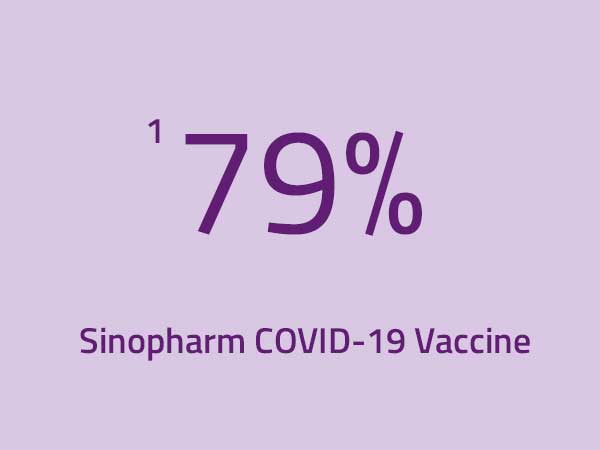

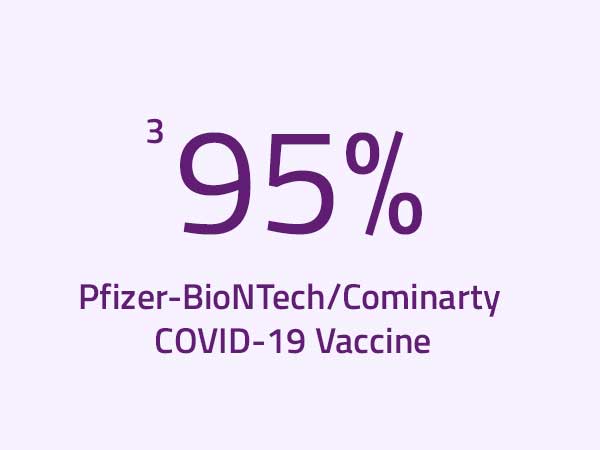

Reference:
1 World Health Organization. 2021. COVID-19 Vaccine Explainer: COVID-19
Vaccine (Vero Cell), Inactivated (Sinopharm). Retrieved from: https://www.who.int/publications/m/item/sinopharm-vero-cell---inactivated-covid-19-vaccine
2 World Health Organization. 2021. COVID-19 Vaccine Explainer: Sinovac CoronaVac [Vero Cell) – Inacctivated, COVID-19 Vaccine. Retrieved from: https://www.who.int/publications/m/item/sinovac-coronavac-vero-cell---inactivated-covid-19-vaccine
3 World Health Organization. 2021. COVID-19 Vaccine Explainer: Pfizer/BioNTech COMIRNATY®, COVID-19 vaccine. Retrieved from: https://www.who.int/publications/m/item/comirnaty-covid-19-mrna-vaccine
4 World Health Organization. 2021. COVID-19 Vaccine Explainer: Moderna mRNA-1273, COVID-19 vaccine. Retrieved from: https://www.who.int/publications/m/item/moderna-covid-19-vaccine-(mrna-1273)

Countries and Territories that Approve the Emergency Use of Sinopharm Vaccine (as of Aug 23)
Angola, Argentina, Bahrain, Bangladesh, Belarus, Belize, Bolivia (Plurinational State of), Brazil, Brunei Darussalam, Cambodia, Cameroon, Chad, China, Comoros, Egypt, Equatorial Guinea, Gabon, Gambia, Georgia, Guyana, Hungary, Indonesia, Iran (Islamic Republic of), Iraq, Jordan, Kyrgyzstan, Lao People's Democratic Republic, Lebanon, Malaysia, Maldives, Mauritania, Mauritius, Mongolia, Montenegro, Morocco, Mozambique, Namibia, Nepal, Niger, North Macedonia, Pakistan, Paraguay, Peru, Philippines, Republic of the Congo, Senegal, Serbia, Seychelles, Sierra Leone, Singapore, Solomon Islands, Somalia, Sri Lanka, Thailand, Trinidad and Tobago, Tunisia, United Arab Emirates, Vanuatu, Venezuela (Bolivarian Republic of), Vietnam, Zimbabwe
Source: COVID-19 Vaccine Tracker by McGill University
There are no restrictions for the vaccine so long as individuals: